Professor
Molecular and Cell Biology Laboratory
Daniel and Martina Lewis Chair

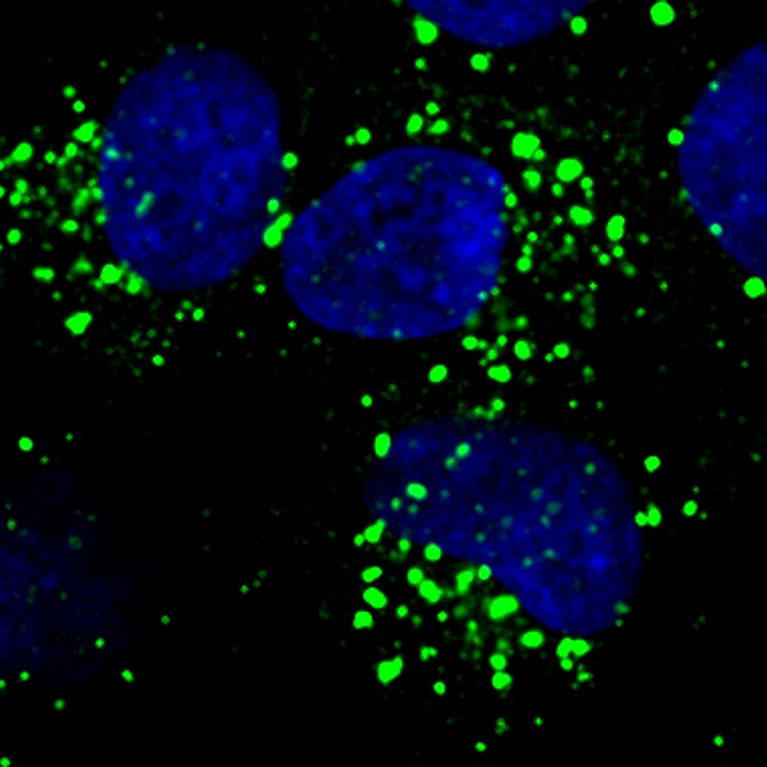
Every organism needs food to survive. For humans this food comes in the form of molecules like glucose, fat and amino acids (the building blocks of proteins). Cells throughout the body metabolize, or break down, these molecules to obtain the building blocks and energy needed to perform life-sustaining tasks. When cellular metabolism goes awry, however, it can lead to metabolic catastrophe and diseases that include cancer, diabetes, and neurodegeneration. By studying how these complex metabolic processes work, scientists can design therapies that target metabolic dysfunction and improve human health.
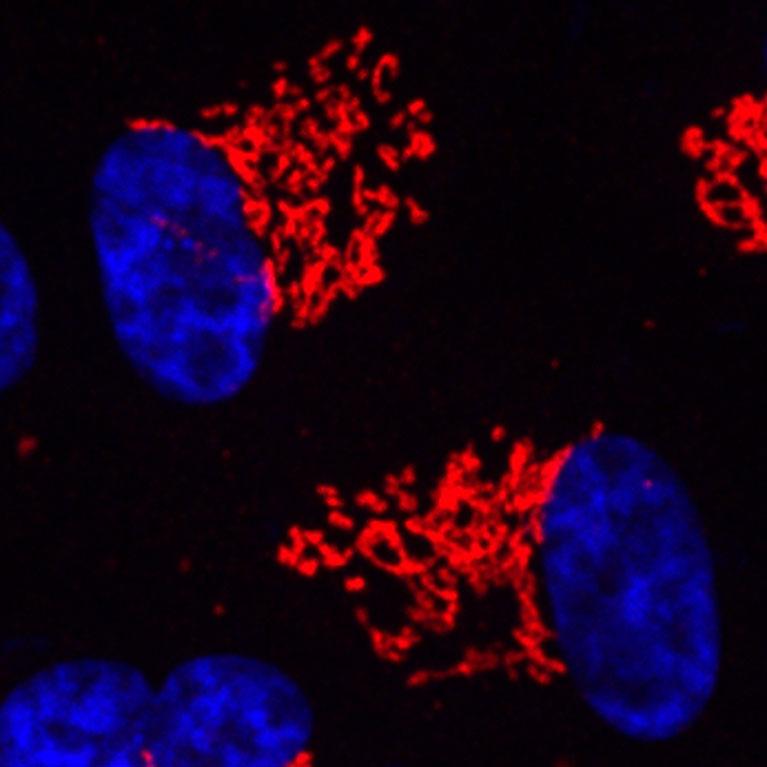
Metallo’s work focuses on mapping these interconnected metabolic networks to uncover disease-causing pathways. Using tracer molecules and advanced mass spectroscopy techniques, his lab identifies how molecules are broken down and re-built, where metabolites end up in the body, and what regulates these processes. Taking this approach, Metallo has made key discoveries about the metabolic pathways that drive cancer progression and macular disease—pathways, which can then be influenced through dietary manipulations or targeted therapies.
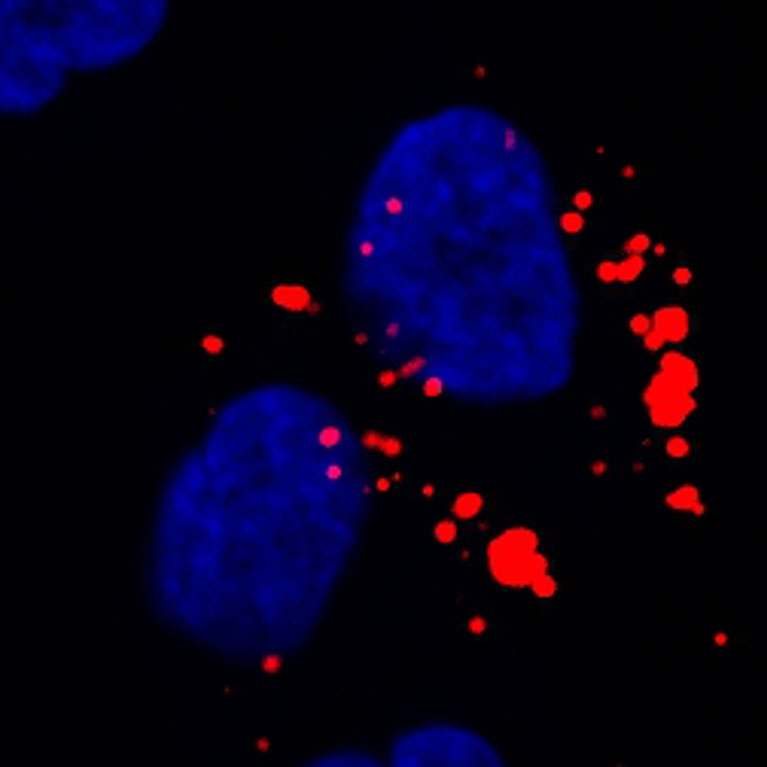
Metallo discovered new insights into the role that two amino acids, serine and glycine, play in tumor progression and demonstrated that restricting dietary serine and glycine in mice alters lipid (fat) metabolism to decrease tumor growth.
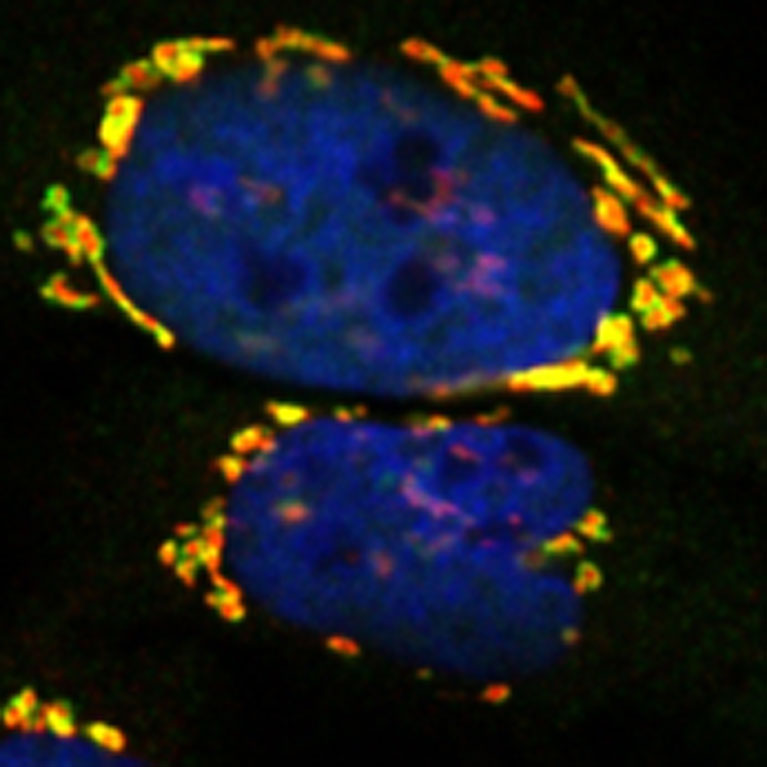
Metallo’s team investigated a metabolic defect that leads to macular telangiectasia type 2. They discovered new biochemical links between peripheral neuropathy and macular disease which may be exploited via diet or pharmacological interventions.
Metallo uncovered the metabolic origin of lipid molecules called branched-chain fatty acids that correlate positively with body fat metabolic activity and are decreased in patients with fatty liver.
BS, Chemical Engineering, University of Pennsylvania
MS, Chemical and Biological Engineering, University of Wisconsin-Madison
PhD, Chemical and Biological Engineering, University of Wisconsin-Madison
Postdoctoral Fellow, Massachusetts Institute of Technology